Learning Outcomes
By the end of this lesson, students will be able to:
i. Explain the relationship between molarity (M) and grams per cubic decimeter (g/dm³), recognizing them as units for expressing solution concentration.
ii. Differentiate between molar mass and molecular mass, understanding their significance in converting between molarity and g/dm³.
iii. Apply the formula for converting molarity to g/dm³: g/dm³ = M × Molar mass (g/mol)
iv. Solve real-world problems involving the conversion between molarity and g/dm³, such as determining the concentration of a solution in g/dm³ for a given molarity or preparing a solution of a specific concentration using both units.
v. Appreciate the practical applications of converting between molarity and g/dm³, particularly in chemistry and environmental science.
Introduction
Molarity and g/dm³ are two common units for expressing the concentration of solutions, each representing the amount of solute per unit volume of solution. While molarity expresses concentration in terms of moles of solute per liter of solution, g/dm³ expresses concentration in terms of grams of solute per cubic decimeter of solution. Understanding the relationship between these units and the ability to convert between them is essential for comprehending various chemical and environmental scenarios.
i. Molarity and g/dm³: A Tale of Two Units
Molarity (M) is a unit of concentration defined as the number of moles of solute per liter of solution. It is represented mathematically as:
Molarity (M) = moles of solute / liters of solution
G/dm³, representing grams per cubic decimeter, is another unit of concentration defined as the number of grams of solute per cubic decimeter of solution. It is expressed mathematically as:
g/dm³ = grams of solute / cubic decimeters of solution
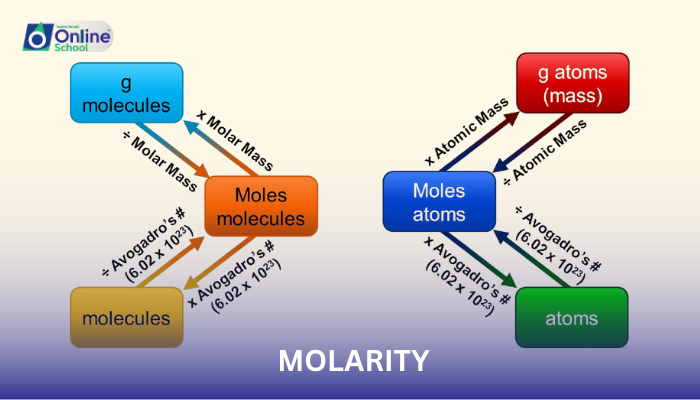
ii. Molar Mass and Molecular Mass: Crucial Links
Molar mass, denoted by M, is the mass of one mole of a substance. It is expressed in grams per mole (g/mol) and is calculated as the sum of the atomic masses of all atoms in the molecular formula of the substance. Molecular mass, also known as the formula mass, is the mass of one molecule of a substance. It is expressed in atomic mass units (amu) and is calculated as the sum of the atomic masses of all atoms in the molecular formula of the substance.
iii. Bridging the Units: The Conversion Formula
The formula for converting molarity (M) to g/dm³ is:
g/dm³ = M × Molar mass (g/mol)
This formula establishes a direct relationship between molarity and g/dm³ by utilizing the molar mass of the solute.
iv. Real-World Applications: Converting between Units in Practice
Converting between molarity and g/dm³ has diverse applications in various fields:
Chemistry: In preparing solutions, converting between molarity and g/dm³ is crucial for accurately measuring the required amount of solute.
Environmental Science: Analyzing the concentration of pollutants in water or air often involves expressing their concentration in both molarity and g/dm³.
Medicine: Determining drug dosages and understanding the concentration of pharmaceutical solutions may require converting between these units.
Food Industry: The concentration of ingredients in food products is often expressed in both molarity and g/dm³.
The ability to convert between molarity and g/dm³ is a valuable tool for comprehending and interpreting solution concentration in various scientific and everyday contexts. By understanding the relationship between these units, the concept of molar mass, and the conversion formula, students gain a deeper appreciation for the quantitative aspects of solutions and their significance in various fields.